About HPV
Global HPV Burden
Human papillomavirus (HPV) refers to a group of over 200 identified viruses. In 90% of individuals, the body controls the infection spontaneously. Persistent infection with high-risk HPV types is the cause of cervical cancer and is associated with cancers of the vulva, vagina, mouth/throat, penis, and anus. In 2019, HPV caused approximately 620,000 cancer cases in women and 70,000 cancer cases in men
| Affected Site | Average Annual Number of Cancers in Sites Commonly Caused by HPV |
% Likely Attributed to HPV | Estimated Number Attributed to HPV |
|---|---|---|---|
| Cervix | 11,959 | 91% | 10,800 |
| Vagina | 898 | 75% | 700 |
| Vulva | 4,418 | 69% | 3,000 |
| Penis | 1,381 | 63% | 900 |
| Anus | 7,854 | 91% | 7,200 |
| Female | 5,363 | 93% | 5,000 |
| Male | 2,491 | 89% | 2,200 |
| Oropharynx | 21,474 | 70% | 15,200 |
| Female | 3,642 | 63% | 2,300 |
| Male | 17,832 | 72% | 12,900 |
| Total | 47,984 | 79% | 37,800 |
| Female | 26,280 | 84% | 21,800 |
| Male | 21,704 | 74% | 16,000 |
The data in this table are derived from population-based cancer registry information from participants in the CDC’s National Program of Cancer Registries (NPCR) and/or the National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) program from 2017 to 2021, covering 98% of the U.S. population.
Worldwide, cervical cancer is the fourth most common cancer among women, with approximately 604,000 cases of invasive cervical cancer diagnosed annually and 342,000 cervical cancer-related deaths.
Types 6 and 11 cause approximately 90% of genital warts.
Types 16 and 18 cause approximately 90% of anal cancers and high-grade anal intraepithelial neoplasia.
Types 16 and 18 are also responsible for about 35–40% of all penile cancers and 70–80% of HPV-positive penile cancers.
It is estimated that the attributable fraction of HPV infection is 29–43% for vulvar cancer, 87% for vulvar intraepithelial neoplasia, 70% for vaginal cancer, and 69–100% for vaginal intraepithelial neoplasia.
Types 16 and 18 account for approximately 35–77% of HPV-positive vulvar cancers, 75–80% of HPV-positive precancerous vulvar lesions, and 60% of HPV-positive vaginal cancers and precancerous vaginal lesions. More than 40 types of HPV infect the anogenital region (3–4).
Virology and Genotypes
Virology:
HPV is a small, non-enveloped, double-stranded circular DNA virus with approximately 7,900 base pairs. DNA sequencing techniques have facilitated HPV genotyping and characterization. Each HPV type is officially distinguished from others by having less than 90% homology in its DNA base pair sequence.
HPV is a small, non-enveloped, capsid virus with an 8-kb circular genome encoding eight genes, including two structural capsid proteins, L1 and L2.
The L1 protein can be expressed recombinantly in cell culture systems and, in the absence of viral genome, forms a virus-like particle (VLP). The L1 VLP is an immunogenic particle used in HPV vaccines. L2 is the minor capsid protein, and together with L1, constitutes the infectious component of HPV.
The virus replication cycle is tightly linked with epithelial differentiation (i.e., keratinocyte maturation).
Initial infection of the basal stem cell occurs following microscopic breaks in the epithelium. Infectious HPV virions appear to attach to the basal cell via tissue-specific heparan sulfate proteoglycans.
Specific viral gene products are transcribed at each level of squamous keratinocyte differentiation. In the superficial layer, the L1, L2, and E4 genes are transcribed to assemble the viral capsid in which the HPV genome is packaged. After desquamation of this short-lived cell, HPV virions are released for the next round of infection (2).
Genotypes:
Different types of HPV show preference for infecting different parts of the body and are therefore associated with different diseases.
Cutaneous
Some HPV types have tropism for skin epithelium and are found in warts such as plantar, common, flat, and butcher’s warts.
HPV types associated with plantar and common warts include types 1, 2, and 4. Flat warts are often caused by types 3 and 10, while butcher’s warts are typically associated with types 7 and 2.
Anogenital epithelium
Some HPV types have tropism for the keratinized skin and mucosal membranes of the anogenital region. Common sites of infection include the penis, scrotum, perineum, anal canal, perianal region, vaginal introitus, vulva, and cervix.
More than 40 mucosal HPV genotypes can infect the genital tract. The clinical manifestations of anogenital diseases vary based on the HPV type.
Benign anogenital warts, most commonly caused by HPV types 6 and 11.
Approximately 15 HPV types are associated with cancer and are referred to as high-risk, oncogenic, or cancer-related types. HPV type 16 is the most common and has the highest risk of progression to cancer.
Cervical transformation zone is not necessary for infection of the female genital tract with oncogenic HPV. Consequently, the prevalence of oncogenic HPV subtypes in the vagina is similar between women who have had a hysterectomy and those who have not.
Similarly, HPV can infect not only the anal canal in the anal transformation zone but also distal areas such as the keratinized skin at the anal verge and perianal region.
HPV type 16 may infect the oral mucosa and is associated with oropharyngeal squamous cell carcinoma.
Respiratory mucosal infection with HPV types 6 and 11 also occurs, particularly in young children and infants.
Genotype-Specific Cancer Risk
Cervical cancer
Head and neck cancers
Anal cancer
Penile cancer
Carcinogenic HPV Types Associated with Cervical Cancer
The majority of cervical cancer cases are attributed to a small number of high-risk HPV genotypes, with HPV-16 and HPV-18 being the most dominant. According to the data, HPV-16 alone accounts for 60.3% of cervical cancer cases, followed by HPV-18 with 10.5%, together contributing to over 70% of the disease burden. Other significant contributors include HPV-45 (6.1%), HPV-33 (3.7%), and HPV-31 (3.6%). Additional types such as HPV-52, 58, 35, 39, 51, 59, 56, and 68 each account for a smaller fraction, ranging between 0.6% to 2.7%. This distribution highlights the importance of vaccines targeting multiple genotypes, particularly those with the highest oncogenic potential, to achieve broader protection against cervical cancer.
Transmission, natural history, and risk factors
HPV is transmitted through skin-to-skin contact. Cutaneous HPV infections are widespread in the general population. Warts occur in 10% of children, with peak incidence between the ages of 12 and 16. Non-genital warts are not limited to children; 3.5% of adults may have non-genital warts at any given time. Common warts account for up to 71% of all cutaneous warts, followed by plantar and flat warts (34% and 4%, respectively). Close personal contact is considered important for transmitting cutaneous warts.
Genital and cervical HPV infections are primarily transmitted through vaginal or anal intercourse. The most consistent predictor of genital HPV infection is sexual activity. Intercourse is an efficient mean of HPV transmission, although it is not strictly necessary for transmission. Other types of contact may also play a role, such as transmission via fingers, sex toys, or other HPV-contaminated genital objects.
Additionally, among heterosexual couples, female-to-male transmission occurs at a higher rate than male-to-female transmission. Transmission in either direction is usually asymptomatic (2).
Most HPV infections, including those with oncogenic genotypes, resolve within 12 months. During cervical HPV infection, low-grade cytologic abnormalities may be detected during screening, but are usually transient.
However, oncogenic HPV infections that persist beyond 12 months are more likely to lead to pre-cancerous or cancerous lesions, though not all persistent infections progress. In the United States, the average age of cytologically detected cervical precancerous lesions is approximately 10 years after the average age of sexual debut.
HPV may enter a latent phase. There is evidence of reactivation of cervical HPV in certain populations, including women living with HIV, older women, and those with a history of cervical dysplasia. However, it remains unclear whether all or only a subset of HPV infections become latent and whether reactivated infections carry a significant risk of cancer (2).
Genital and cervical HPV infections are primarily transmitted via genital-genital or anal-genital contact. Sexual activity remains the strongest predictor of genital HPV infection. The following findings have been consistently observed in studies of young women:
- The risk of cervical and vaginal HPV infection in women is directly associated with the number of male sexual partners and the number of female partners of those male partners.
- Similar to other sexually transmitted infections, sex with a new partner poses a greater risk than with a long-term partner.
- Both vaginal and anal intercourse are major risk factors for HPV infection.
- While vaginal intercourse is not not required for transmission, HPV prevalence is significantly lower among virgins.
- Anal intercourse is likely an efficient route for HPV spread to the anal canal, although, like vaginal intercourse, it is not required for transmission.
- Other routes such as contact via fingers, sex toys, or other HPV-contaminated genital areas may also contribute.
Among heterosexual couples, type-specific concordance (i.e., both partners being infected with the same HPV type) is common, with approximately 25% observed in one case-control study. Transmission in both directions is generally asymptomatic.
Several studies in women have shown that the presence of anti-HPV antibodies—indicative of past infection—is associated with a reduced risk of subsequent infection with the same type, particularly type 16, suggesting potential for protective immunity following natural infection.
However, the strength and duration of such immunity remain uncertain, and many women do not develop detectable antibodies after infection.
Correct and consistent condom use reduces the risk of HPV infection, but condoms do not fully prevent transmission due to the possibility of skin-to-skin contact.
Although intrauterine device (IUD) use has been associated with a lower risk of cervical cancer, it does not appear to affect the acquisition or clearance of genital HPV infection.
Common risk factors for HPV infection in men include HIV infection, current and past sexual behaviors, number of sexual partners, lack of condom use, prior STIs, race, ethnicity, and circumcision status.
Studies indicate that uncircumcised men have slower HPV clearance than circumcised men.
Additionally, among men who have sex with men (MSM), risk factors for HPV positivity include younger age at sexual debut, high number of receptive anal sex partners, and less condom use.
HPV anal infection is more prevalent among HIV-positive men.
Risk factors for anal HPV infection include high lifetime number of female sexual partners, shorter duration of current sexual relationship, prior genital HPV infection, and previous hepatitis B virus infection.
Risk factors for cancer progression include the oncogenic potential of the HPV genotype, immune status, presence of other STIs, number of births, young age at first pregnancy, hormonal contraceptive use, and smoking (5).
Global and Regional HPV Burden and Epidemiology
A meta-analysis of studies involving over 150,000 women with normal cervical cytology showed that the global point prevalence of HPV is approximately 10%. The highest regional prevalence was reported in Africa, where 22% of women had evidence of HPV infection. Similar to the United States, it is expected that the global epidemiology of HPV infection will shift with the implementation of routine HPV vaccination in most countries and improved access to vaccines.
However, global vaccine uptake among eligible girls remains low. In 2020, global coverage with two doses of the vaccine was only 13%. The most common HPV types worldwide are types 16 and 18, both of which are preventable through vaccination. Nevertheless, geographical variation in the distribution of HPV genotypes appears to play a significant role.
The global prevalence of HPV in men is estimated to be around 30–35%. A consistent finding across populations is the association between increased sexual activity and high-risk HPV genotypes.
In U.S. men, based on data collected from NHANES participants during 2013–2014, where penile swabs were used for HPV DNA testing, the estimated point prevalence of genital HPV infection was 45% for all types and 25% for high-risk types. These figures represent only point prevalence estimates, and the lifetime risk of acquiring genital HPV infection is significantly higher.
More than 42 million Americans are infected with HPV types known to cause disease. About 13 million Americans, including adolescents, become newly infected each year.
In the United States, 36,500 individuals annually (including both women and men) are diagnosed with cancers attributed to HPV infection. Although cervical cancer is the most well-known HPV-related cancer, several other cancers are also caused by HPV.
HPV vaccination can prevent more than 90% of HPV-related cancers—an estimated 33,700 preventable cases per year in the U.S. Despite existing screening programs, HPV causes approximately 11,000 cases of cervical cancer annually in the U.S., leading to around 4,000 cervical cancer deaths each year.
It is also estimated that 196,000 cases of cervical precancer are diagnosed annually. Treatments for cervical cancer and precancerous lesions may sometimes impair fertility. Each year in the U.S., approximately:
- 14,000 cases of oropharyngeal cancer,
- 6,500 cases of anal cancer,
- 3,500 cases of vulvar and vaginal cancers, and
- 900 cases of penile cancer are reported.
Globally, HPV types 16 and 18 are responsible for nearly 70% of cervical cancer cases, while HPV types 6 and 11 cause 90% of genital warts.
The highest prevalence of cervical HPV infection among women is reported in:
- Sub-Saharan Africa (24%)
- Latin America and the Caribbean (16%)
- Eastern Europe (14%)
- Southeast Asia (14%)
Evidence suggests that HPV prevalence is higher among certain groups, including:
- Women living with HIV
- Men who have sex with men (MSM)
- Immunocompromised individuals
- Those co-infected with other sexually transmitted infection (STI)
- Individuals on immunosuppressive therapies
- Children who have been sexually abused
Globally, it is estimated that in 2019, 620,000 new cancer cases in women and 70,000 in men were attributable to HPV. Cervical cancer was the fourth leading cause of cancer incidence and mortality among women in 2022, with approximately 660,000 new cases and 350,000 deaths worldwide.
More than 90% of HPV-related cancers in women are cervical cancers. The highest incidence and mortality from cervical cancer are observed in low- and middle-income countries, reflecting major disparities in access to national HPV vaccination, screening, cervical cancer treatment services, and underlying social and economic determinants.
The results of the meta-analysis for pooled estimated prevalence, along with reported prevalence for all HPV types as well as HPV16, HPV18, and other HPV genotypes among healthy women by province, are shown in Figure 1 (9). In Iran, the highest overall HPV prevalence is in Zanjan and Kerman provinces (32.3% and 24%, respectively). The highest prevalence of HPV16 is in Bushehr and Khuzestan provinces (29.8% and 25.3%, respectively). HPV18 prevalence is highest in Tehran (8.9%), and the highest prevalence of other HPV genotypes is reported in Khuzestan (54.2%).
HPV and Cancer: Cervical, Anal, Penile, Oropharyngeal
Globally, cervical cancer is the fourth most common cancer among women, with nearly 604,000 new cases of invasive cervical cancer and 342,000 cervical cancer-related deaths each year. The association between HPV and cervical cancer is well established: almost all cervical cancer cases are attributable to HPV infection, with types 16 and 18 responsible for most of them.
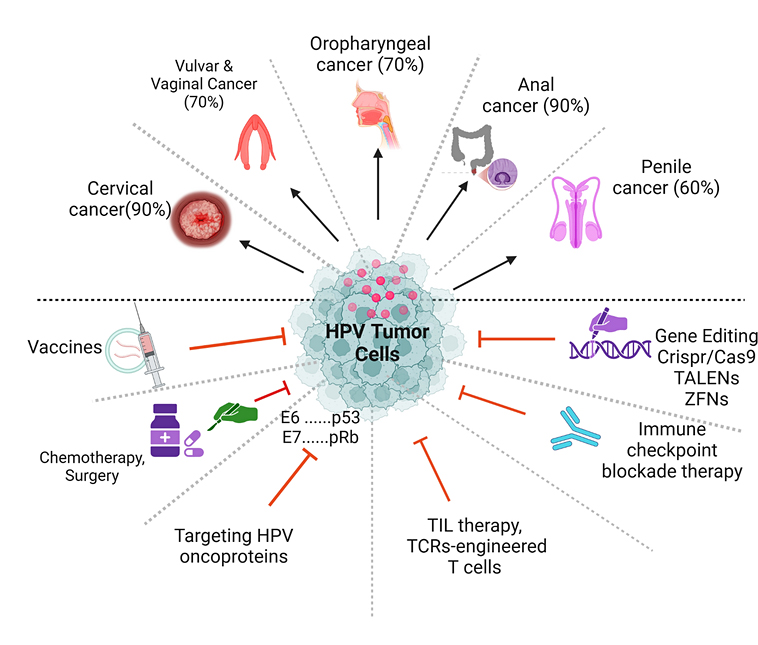
Vulvar and vaginal cancers are rare worldwide. Unlike cervical cancer, not all cancers of the external genital tract are associated with HPV infection. The HPV-attributable fraction is estimated at 29–43% for vulvar cancer, 87% for vulvar intraepithelial neoplasia, 70% for vaginal cancer, and 69–100% for vaginal intraepithelial neoplasia.
HPV types 16 and 18 are responsible for approximately 35–77% of HPV-positive vulvar cancers, 75–80% of HPV-positive precancerous vulvar lesions, and 60% of HPV-positive vaginal cancers and precancerous vaginal lesions.
In contrast to HPV-negative external genital tract cancers, HPV-associated vulvar cancers occur at a younger age, display basaloid rather than keratinized histology, lack p53 mutations, and are linked to sexual risk factors. HPV-associated vaginal cancers share similar characteristics, but overall, vaginal cancer is more likely to be HPV-related.
Anal cancer is relatively uncommon in the general population worldwide, although its incidence is rising in some high-resource regions, including the United States. In the U.S., anal cancer is now more common than cervical cancer among white women aged 65 years and older. HPV types 16 and 18 cause about 90% of anal cancers and high-grade anal intraepithelial lesions. Women are more likely than men to develop anal cancer; however, the highest rates are seen in men who have sex with men, especially those living with HIV.
HPV infection also plays a role in the pathogenesis of squamous cell carcinoma of the head and neck. Similar to penile and vulvar cancer, oropharyngeal cancer can be divided into HPV-related and HPV-unrelated categories.
HPV-related oropharyngeal cancers most commonly arise in the oropharynx (the part of the throat behind the mouth), particularly the base of the tongue and tonsils. HPV is also associated with laryngeal cancer. HPV-related oropharyngeal cancers tend to occur in younger populations compared with HPV-unrelated cases and are linked to sexual risk factors. In contrast, HPV-unrelated oropharyngeal cancers are primarily associated with alcohol and tobacco use and often have p53 mutations.
In the United States, the incidence of HPV-related oropharyngeal cancers is increasing, while the incidence of HPV-unrelated cases is decreasing. Currently, HPV-related oropharyngeal cancers are more common than HPV-unrelated ones. In 2015, oropharyngeal squamous cell carcinoma became the most common HPV-associated cancer. In an age- and sex-matched case–control study of 130 patients newly diagnosed with head and neck squamous cell carcinoma, oropharyngeal malignancy was associated with high-risk sexual behaviors, oropharyngeal HPV infection, and HPV-16 seropositivity.
Penile cancer is rare globally, though it accounts for up to 10% of male cancers in some regions of Africa, South America, and Asia. Unlike cervical cancer, not all cancers of the external male genital tract are associated with HPV infection. HPV types 16 and 18 cause about 35–40% of all penile cancers and 70–80% of HPV-positive penile cancers. Compared with HPV-negative external genital cancers, HPV-positive penile cancers occur at a younger age, exhibit basaloid rather than keratinizing pathology, lack p53 mutations, and are associated with sexual risk factors.

