
Overview
PapilloGuard4® is a quadrivalent, recombinant HPV vaccine developed by Nivad Pharmed Salamat for the prevention of HPV-related diseases in both women and men. It specifically targets HPV types 6, 11, 16, and 18, which are among the most clinically significant genotypes:
- HPV 16 and 18 are high-risk oncogenic types, collectively responsible for about 70% of cervical cancers and a large proportion of anal, vulvar, vaginal, penile, and oropharyngeal cancers.
- HPV 6 and 11 are low-risk types that cause about 90% of genital warts and are most common types involved in recurrent respiratory papillomatosis.
By addressing both high-risk and low-risk types, PapilloGuard4® provides broad-spectrum protection. Its role is not therapeutic but prophylactic, offering the greatest benefit when administered before exposure to HPV, ideally prior to sexual debut. The vaccine is recommended for individuals aged 9 to 45 years, providing long-lasting protection when administered early and according to the complete dosing schedule.
Composition
PapilloGuard4® is formulated with recombinant L1 proteins from HPV types 6, 11, 16, and 18, combined with an advanced adjuvant system called “Adjuvant System 04 (AS04)” that enhances immune response and durability. Alongside these active antigens, selected excipients ensure stability, safety, and efficacy of the vaccine. This section details the precise formulation supporting its preventive role against HPV-related diseases.
Active ingredients
-
HPV type 6 L1 protein20 µg
-
HPV type 11 L1 protein40 µg
-
HPV type 16 L1 protein40 µg
-
HPV type 18 L1 protein20 µg
Excipients
3-O-desacyl-4′-monophosphoryl lipid A (MPL), aluminium hydroxide (Al(OH)₃), sodium chloride (NaCl), sodium dihydrogen phosphate monohydrate, disodium hydrogen phosphate heptahydrate, and water for injection (WFI).
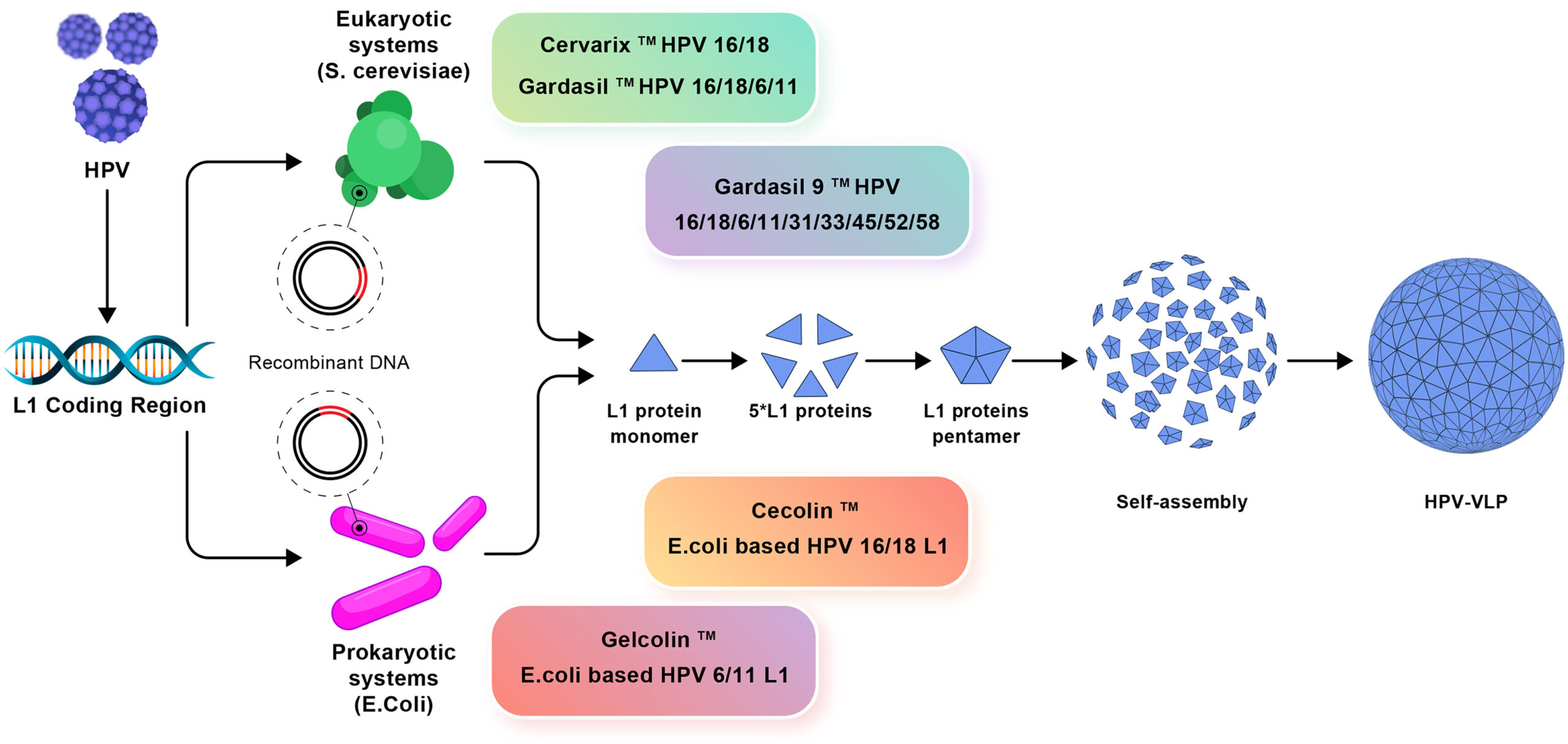
Mechanism of Action
PapilloGuard4® contains recombinant L1 proteins of human papillomavirus (HPV) types 6, 11, 16, and 18. These proteins stimulate the immune system to generate antibodies against HPV, thereby preventing cervical, vulvar, vaginal, and anal cancers, cervical adenocarcinoma, precancerous lesions, as well as genital warts caused by HPV.
Innovation in the Adjuvant Formulation
The use of the AS04 adjuvant system, composed of aluminium hydroxide and monophosphoryl lipid A (MPL), represents a key innovation in the design of HPV vaccines. This advanced combination:
Enhances immunogenicity compared to conventional aluminium-based adjuvants.
Provides longer durability of antibody responses, ensuring sustained protection.
Increases the potential for cross-protection against HPV types beyond 6, 11, 16, and 18.
This innovative adjuvant system strengthens the immunogenicity and long-term protective profile of PapilloGuard4®, distinguishing it from vaccines that rely solely on traditional aluminium adjuvants.

